Does If4+ Have a Dipole Moment
Which direction does it point. It is denoted by the Greek letter µ.

Be Sure To Answer All Parts Which Of The Following Ions Possess A Dipole Moment A Clf2 B Clf2 Brainly Com
Does SiF4 have polar bondsStructure of SiF4 is a regular tetrahedron any regular geometry has net zero dipole moment as all individual.

. Question posted by Student Graphs are really useful in Physics and the following pages show you the shapes of some of the most important graphs. What are the formal charges here. Consider the molecules PF5 BrF3 BrF5 and SF4.
Some types of molecules that do not possess an intrinsic electric dipole moment can be given. The major resonance structure has one double bond. What is polar and non-polar.
The molecular structure of IF4- is. It is measured in Debye units denoted by D. SiF4 Dipole Moment.
The nonpolar bond formed between two atoms tends to have equal electronegativity. The shape of nonpolar molecules are symmetric. The dipole moments cancel each other.
The dipole moment is defined as the products of induced charge and distance of separation between the atoms. Answer 1 of 10. Hence it does not have a permanent dipole moment.
Does ammonia have a dipole moment. It is given as Dipole moment Charge Q distance of separation d Its unit is Debye and denoted by D. It is a colorless non-flammable gaseous compound which falls under the category of haloalkane a combination of halogen and alkane.
Answer IF4- is Nonpolar. Do you want to see how step-by-step Q A looks like. H2 is a non-polar molecule as there is no difference in electronegativity between H-H.
CHEMWOK Predict the molecular structure bond angles and polarity dipole moment for each of the following. Not necessarily the same value for each graph. A ClF2 has a dipole moment has no dipole moment cannot be determined b ClF2 has a dipole moment has no dipole moment cannot be determined c IF4 has a dipole moment has no dipole moment cannot be determined d IF4 has a dipole moment has no dipole moment cannot be determined Can someone.
Do you think a bent molecule has a dipole moment. 1 D 333564 10-30 Cm where C is Coulomb and m denotes a meter. Try drawing the resonance hybrid structure and consider dipole moment vector diagrams like these when determining how to draw your dipole moment vector.
A scientist discovered an unknown organism in. Formula Molecular structure Bond angles IF3 KrF4 BH3 Dipole moment XeF CBr4 Submit. Which of the following ions possess a dipole moment.
Is h2 dipole dipole or dispersion. A dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. Furthermore does silicon tetrafluoride have a dipole moment.
SiF4 is tetrahedral so that the individual dipoles on the Si-F bonds cancel and the molecule has no dipole moment. - has a permanent dipole. CHEMWOK Predict the molecular structure bond angles and polarity dipole moment for each of the following.
Its dipole moment is the net dipole moment resulting from three individual bond moments. Does if4 have a dipole moment. The hybridization of the central atom in NO3- is.
This problem has been solved. Some of the examples of nonpolar molecules are Hexane NO2. The dipole moments must not cancel out but I am having a hard time understanding why.
Which does not have a molecular dipole moment. A predicting the time of sunrise based on data on position of earth b predicting the date of the moon phases based on data on position of earth c predicting eclipses based on the position of the sun and the moon d predicting future events in a persons life based on the position of the moon. Chemistry questions and answers.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Symmetrical molecules are usually nonpolar. Does cf4 have a dipole moment Carbon tetrafluoride or tetrafluoromethane is the simplest fluorocarbon with very high bond strength.
BrF5 or bromine pentafluoride has a square pyramidal structure as in the first figure. The molecular structure of IF4- is. Why does IF4- not have a dipole moment.
Which of the following is the correct organizational scale for the economic community of west african states ecowas. C3Cl2H2 is polar but why. See the answer See the answer See the answer done loading.
Ammonia has a dipole moment of 146D. In the molecule C2H4 the valence orbitals of the carbon atoms are assumed to be. A molecule which contains polar bonds will always have a dipole moment.
Molecule is sp 3d thus a seesaw structure bent because of lp-lp repulsion and thus having a net dipole moment. CF4 is symmetrical and nonpolar. You can check out the reason for the.
A ClF2 has a dipole moment has no dipole moment cannot be determined b ClF2 has a dipole moment has no dipole moment cannot be determined c IF4 has a dipole moment has no dipole moment cannot. Some types of molecules that do not possess an intrinsic electric dipole moment can be given one by an external electric field in a process called charge separation or polarization. These molecules always have zero dipole moment.
Whenever a set of equivalent tetrahedral atomic orbitals is. Does BrF5 have a dipole moment. The molecule is tetrahedral and the vector sum of the four individual bond dipoles is zero.
Hence it does not have a permanent dipole moment. With a net dipole moment of zero the molecule is nonpolar. The dipole moments must not cancel out but I am having a hard time understanding why.
If a molecule has polar bonds does it also have Dipole-Dipole IMFs. What is the hybridization of the nitrogen atom. Which of the following ions possess a dipole moment.
1D 33356410-30 Cm where C is Coulomb and m is meter. What kind of bond is SiF4. The dipole moments cancel each other.
The hybridization of the phosphorus atom in the cation PH2 is. C m and k are constants. In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Which of the following represents a function brainly. If we consider PCl5 which has a trigonal bi-pyramidal structure there is no net dipole moment as the molecule is completely symmetric around the central phosphorus atom. There is a 90 chance that we have answers for your questions.

Dipole Moments Mcc Organic Chemistry
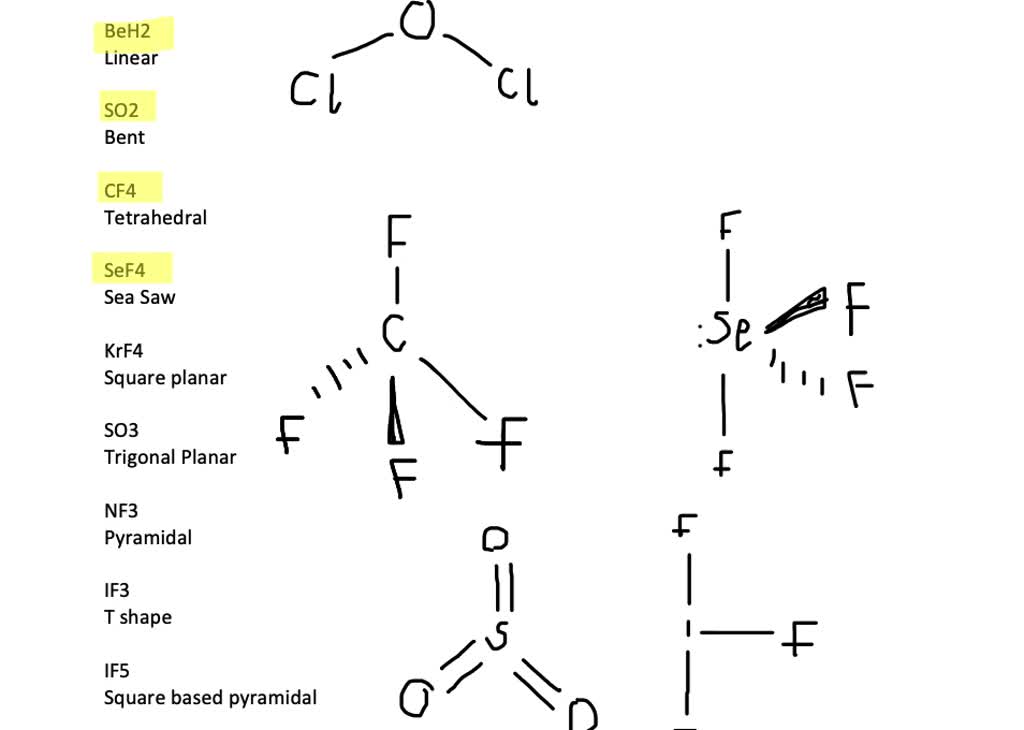
Solved Which Of The Following Ions Possess A Dipole Moment A Cif2 B Cif2 C If4 D If4 Please Draw Each Lewis Dot And Explain Why It Has Or Not

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube

Molecular Dipole And Polarity Practice Problems Molecular Chemistry Molecules
Chapter 9 Molecular Geometry Ppt Download

Water Molecular Dipole By Vector Sum Chemistry Lessons Chemistry Molecular

Dipole Moment Calculation In This Moment Mcat The Unit

Dipole Moment And Symmetry Affect Boiling Point And Melting Point Organic Chemistry Chemistry Chemistry Notes

In Co2 Carbon Dioxide Dipole Moments Cancel Chemistry Science Chemistry Molecular

How The Molecular Dipole Moment Affects The Physical Properties Molecular Chemistry Physics

Dipole Moments Mcc Organic Chemistry

Dipole Moments Mcc Organic Chemistry

Dipole Moments Mcc Organic Chemistry

Dipole Moment Bond Moment Group Moment And Influence Of Dipole Moment Chemsolve Net Organic Chemistry Books Covalent Bonding Physical And Chemical Properties
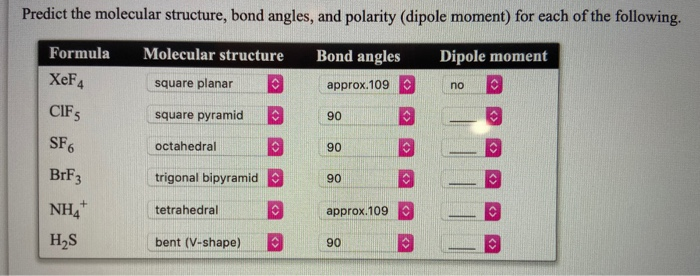
Solved Predict The Molecular Structure Bond Angles And Chegg Com

Dipole Moment Of Bef2 Covalent Bonding Chemical Bond Ionic Bonding

Dipole Moments Mcc Organic Chemistry

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube

Comments
Post a Comment